Section: Research Program
Geometry and machine learning for 3D interaction prediction
Participants : Julie Bernauer, Jean-Marc Steyaert, Christine Froidevaux, Adrien Guilhot-Gaudeffroy, Amélie Héliou.
The biological function of macromolecules such as proteins and nucleic acids relies on their dynamic structural nature and their ability to interact with many different partners. This is specially challenging as structure flexibility is key and multi-scale modelling [47] , [57] and efficient code are essential [61] .
Our project covers various aspects of biological macromolecule structure and interaction modelling and analysis. First protein structure prediction is addressed through combinatorics. The dynamics of these types of structures is also studied using statistical and robotics inspired strategies. Both provide a good starting point to perform 3D interaction modelling, accurate structure and dynamics being essential. Modelling is then raised to the cell level by studying large protein interaction networks and also the dynamics of molecular pathways.
Our group benefits from a good collaboration network, mainly at Stanford University (USA), Hkust (Hong-Kong) and McGill (Canada). The computational expertise in this field of computational structural biology is represented in a few large groups in the world (e.g. Pande lab at Stanford, Baker lab at U.Washington) that have both dry and wet labs. We also contributed to the Capri experiment organized by leading member of an international community we have been involved in for some time [56] . At Inria, our interest for structural biology is shared by the Abs project-team. A work by D. Ritchie in the Orpailleur project-team (see [44] led to a joint publication with T. Bourquard and J. Azé. Our activities are however now more centered around protein-nucleic acid interactions, multi-scale analysis, robotics inspired strategies and machine learning than protein-protein interactions, algorithms and geometry. We also shared a common interest for large biomolecules and their dynamics with the Nano-D project team and their adaptative sampling strategy. As a whole, we contribute to the development of geometric and machine learning strategies for macromolecular docking.
Combinatorial models for the structure of proteins
Protein structure prediction has been and still is extensively studied. Computational approaches have shown interesting results for globular proteins but transmembrane proteins remain a difficult case.
Transmembrane beta-barrel proteins (TMB) account for 20 to 30% of identified proteins in a genome but, due to difficulties with standard experimental techniques, they are only 2% of the RCSB Protein Data Bank. As TMB perform many vital functions, the prediction of their structure is a challenge for life sciences, while the small number of known structures prohibits knowledge-based methods for structure prediction.
As barrel proteins are strongly structured objects, model based methodologies are an interesting alternative to these conventional methods. Jérome Waldisphül's thesis at Lix had opened this track for the common case where a protein folds respecting the order of the sequence, leaving a structure where each strand is bound to the preceding and succeeding ones. The matching constraints were expressed by a grammatical model, for which relatively simple dynamic programming schemes exist.
However, more sophisticated schemes are required when the arrangements of the strands along the barrel do not follow their order in the sequence, as it is the case for Greek key or Jelly roll motifs. The prediction algorithm may then be driven by a permutation on the order of the bonded strands. In his thesis [75] , Van Du Tran developed a methodology for compiling a given permutation into a dynamic programming scheme that may predict the folding of sequences into the corresponding TMB secondary structure. Polynomial complexity upper bounds follow from the calculated DP scheme. Through tree decompositions of the graph that expresses constraints between strands in the barrel, better schemes were investigated in [75] .
The efficiently obtained 3D structures provide a good model for further 3D and interaction analyses.
3D interaction prediction
To better model complexes, various aspects of the scoring problem for protein-protein docking need being addressed [56] . It is also of great interest to introduce a hierarchical analysis of the original complex three-dimensional structures used for learning, obtained by clustering.
A protein-protein docking procedure traditionally consists in two successive tasks: a search algorithm generates a large number of candidate solutions, and then a scoring function is used to rank them in order to extract a native-like conformation. We demonstrated that, using Voronoi constructions and a defined set of parameters, we could optimize an accurate scoring function and interaction detection [46] . We also focused on developing other geometric constructions for that purpose: being related to the Voronoi construction, the Laguerre tessellation was expected to better represent the physico-chemical properties of the partners. It also allows a fast computation without losing the intrinsic properties of the biological objects. In [49] , we compare both constructions. We also worked on introducing a hierarchical analysis of the original complex three-dimensional structures used for learning, obtained by clustering. Using this clustering model, in combination with a strong emphasis on the design of efficient complex filters collaborative filtering, we can optimize the scoring functions and get more accurate solutions [50] .
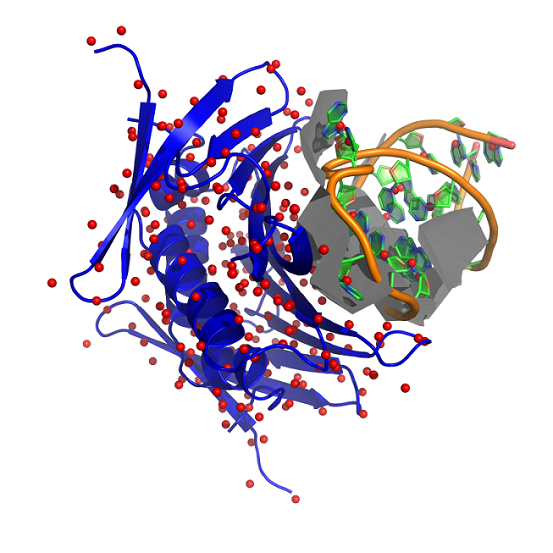
These techniques have been extended to the analysis of protein-nucleic acid complexes : developments and tests are performed by A. Guilhot (See figure 2 ) in his PhD thesis.